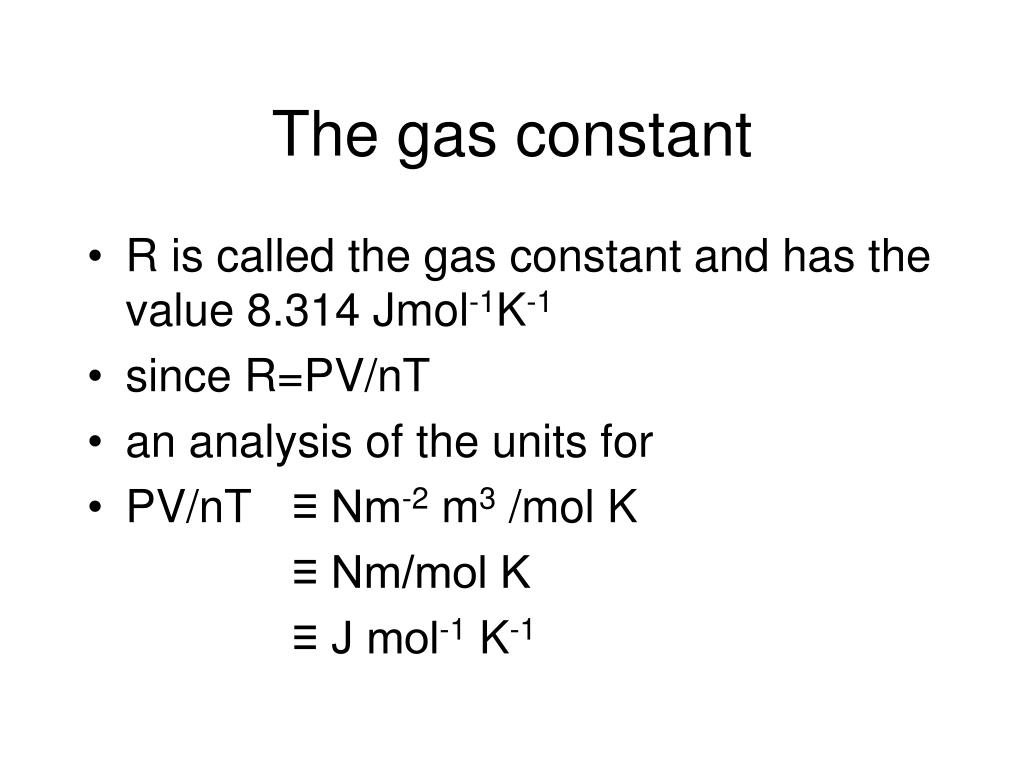
Gas Constant Wiki . Since there is avogadro’s number of molecules per mole, we. The boltzmann constant (k or k b) is a physical constant. We also need the gas constant expressed per molecule rather than per mole. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. [1] it relates the average kinetic energy of. It is defined to be 1.380 649 × 10 −23 j/k. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic.
from www.slideserve.com
[1] it relates the average kinetic energy of. Since there is avogadro’s number of molecules per mole, we. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. It is defined to be 1.380 649 × 10 −23 j/k. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. The boltzmann constant (k or k b) is a physical constant. We also need the gas constant expressed per molecule rather than per mole.
PPT Moles and the Ideal Gas equation PowerPoint Presentation, free
Gas Constant Wiki R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. Since there is avogadro’s number of molecules per mole, we. We also need the gas constant expressed per molecule rather than per mole. The boltzmann constant (k or k b) is a physical constant. [1] it relates the average kinetic energy of. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. It is defined to be 1.380 649 × 10 −23 j/k. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law.
From tristinkruwchandler.blogspot.com
Boltzmann Constant and Avogadro's Number TristinkruwChandler Gas Constant Wiki It is defined to be 1.380 649 × 10 −23 j/k. [1] it relates the average kinetic energy of. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. The boltzmann constant (k or k b) is a. Gas Constant Wiki.
From dewwool.com
Gas Constant DewWool Gas Constant Wiki Since there is avogadro’s number of molecules per mole, we. The boltzmann constant (k or k b) is a physical constant. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. [1] it relates the average kinetic energy of. Universal gas constant (r), fundamental physical constant arising in the formulation. Gas Constant Wiki.
From sciencenotes.org
Ideal Gas Constant (R) Universal Gas Constant Gas Constant Wiki R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. Since there is avogadro’s number of molecules per mole, we. The boltzmann constant (k or k b) is a physical constant. [1] it relates the average kinetic energy. Gas Constant Wiki.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID21353 Gas Constant Wiki It is defined to be 1.380 649 × 10 −23 j/k. Since there is avogadro’s number of molecules per mole, we. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. We also need the gas constant expressed per molecule rather than per mole. Universal gas constant (r), fundamental physical constant arising in the. Gas Constant Wiki.
From antiluli.weebly.com
Gas constant iunit antiluli Gas Constant Wiki If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. It is defined to be 1.380 649 × 10 −23 j/k. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. The boltzmann constant (k or k b) is a physical constant. We also. Gas Constant Wiki.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Gas Constant Wiki Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. [1] it relates the average kinetic energy of. The boltzmann constant (k or k b) is a physical constant. It is defined to be 1.380 649 × 10. Gas Constant Wiki.
From www.youtube.com
Universal Gas Constant YouTube Gas Constant Wiki The boltzmann constant (k or k b) is a physical constant. We also need the gas constant expressed per molecule rather than per mole. Since there is avogadro’s number of molecules per mole, we. It is defined to be 1.380 649 × 10 −23 j/k. R) is the proportionality constant between the product of pressure and volume, pv, and the. Gas Constant Wiki.
From www.slideserve.com
PPT Gas Stoichiometry Ideal Gas Law PowerPoint Presentation, free Gas Constant Wiki [1] it relates the average kinetic energy of. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. It is defined to be 1.380 649 × 10 −23 j/k. The boltzmann constant (k or k b) is a. Gas Constant Wiki.
From www.coursehero.com
DETERMINATION OF THE GAS CONSTANT INTRODUCTION The ideal gas law Gas Constant Wiki We also need the gas constant expressed per molecule rather than per mole. Since there is avogadro’s number of molecules per mole, we. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. [1] it relates the average. Gas Constant Wiki.
From www.slideserve.com
PPT Electrochemistry and Corrosion PowerPoint Presentation, free Gas Constant Wiki It is defined to be 1.380 649 × 10 −23 j/k. The boltzmann constant (k or k b) is a physical constant. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. Since there is avogadro’s number of molecules per mole, we. We also need the gas constant expressed per. Gas Constant Wiki.
From studylib.net
Experiment 7 The Gass Constant Gas Constant Wiki Since there is avogadro’s number of molecules per mole, we. We also need the gas constant expressed per molecule rather than per mole. The boltzmann constant (k or k b) is a physical constant. [1] it relates the average kinetic energy of. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. If the. Gas Constant Wiki.
From www.researchgate.net
1 Atmospheric gases constant concentrations. Download Table Gas Constant Wiki R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. The boltzmann constant (k or k b) is a physical constant. Since there is avogadro’s number of molecules per mole, we. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. We also need. Gas Constant Wiki.
From www.slideserve.com
PPT Gas Law PowerPoint Presentation, free download ID5965812 Gas Constant Wiki If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. We also need the gas constant expressed per molecule rather than per mole. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. [1] it relates the average kinetic energy of. It is defined. Gas Constant Wiki.
From www.diplomageeks.com
Gas constant, universal gas constant, SI units of UGS, value of UGS Gas Constant Wiki The boltzmann constant (k or k b) is a physical constant. We also need the gas constant expressed per molecule rather than per mole. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas law. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume.. Gas Constant Wiki.
From stock.adobe.com
PV = nRT Ideal Gas Law Brings Together Gas Properties. The Most Gas Constant Wiki [1] it relates the average kinetic energy of. It is defined to be 1.380 649 × 10 −23 j/k. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. Since there is avogadro’s number of molecules per mole, we. Universal gas constant (r), fundamental physical constant arising in the formulation of the ideal gas. Gas Constant Wiki.
From www.thoughtco.com
Chemistry Definition of Gas Constant (R) Gas Constant Wiki It is defined to be 1.380 649 × 10 −23 j/k. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. [1] it relates the average kinetic energy of. The boltzmann constant (k or k b) is a physical constant. If the number of gas molecules and the temperature remain constant, then the pressure. Gas Constant Wiki.
From www.bioblast.at
Gas constant Bioblast Gas Constant Wiki We also need the gas constant expressed per molecule rather than per mole. Since there is avogadro’s number of molecules per mole, we. [1] it relates the average kinetic energy of. The boltzmann constant (k or k b) is a physical constant. R) is the proportionality constant between the product of pressure and volume, pv, and the thermodynamic. Universal gas. Gas Constant Wiki.
From www.slideserve.com
PPT Moles and the Ideal Gas equation PowerPoint Presentation, free Gas Constant Wiki [1] it relates the average kinetic energy of. If the number of gas molecules and the temperature remain constant, then the pressure is inversely proportional to the volume. Since there is avogadro’s number of molecules per mole, we. It is defined to be 1.380 649 × 10 −23 j/k. R) is the proportionality constant between the product of pressure and. Gas Constant Wiki.